Organic peroxides have received considerable attention in pharmacy, biochemistry and food chemistry because of their close relationship with drug design, cell damage, and food safety. Organic peroxides also play as key reactive intermediates in synthetic chemistry. However, the established methods have more-limited scope. Therefore, new peroxidation methods are highly desirable and valuable.
In 2011, Prof. Li’s group reported an iron-catalyzed three-component acylation-peroxidation reaction of alkene, aldehyde and hydroperoxide (Scheme 1,J. Am. Chem. Soc. 2011, 133, 10756-10759; Tetrahedron Lett. 2013, 54, 6337-6340). This methodology allows a carbonyl group and a peroxy group to add across C=C bonds of styrene derivatives selectively. The gram-scale experiment was also performed to show the practicality and generality of this strategy.
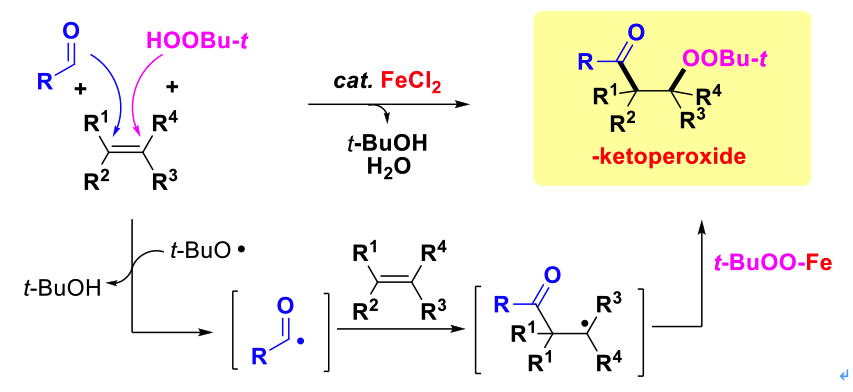
Scheme 1: Iron-catalyzed acylation-peroxidation of alkenes
Besides, the generated peroxides could be efficiently transformed into multi-substituted carbonyl epoxides with excellent diasteroselectivity in the presence of an amine catalyst. Furthermore, a library of α,β-epoxy-γ-butyrolactones were readily synthesized stereospecifically with those densely functionalized epoxides as precursors under mild reductive conditions. (Scheme 2,Chem. Asian J. 2013, 8, 359-363).
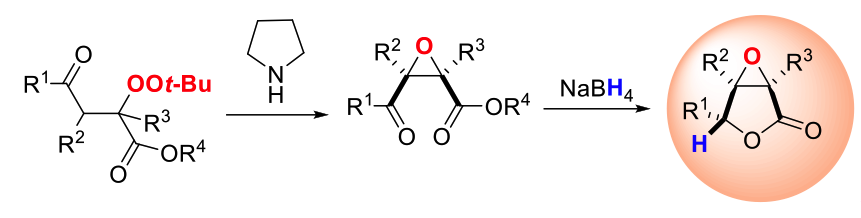
Scheme 2: Synthesis of multi-substituted carbonyl epoxides andα,β-epoxy-γ-butyrolactones
Clavilactones A-E, isolated from cultures of the Basidiomycetous fungus Clitocybe clavipes,1 are antifungal candidates and potent kinase inhibitors against Ret/ptc1and epidermal growth factor receptor (EGF-R) tyrosine kinases. The clavilactones have novel structures with a ten-membered carbocycle fused to an α,β-epoxy-γ-butyrolactones and a benzoquinone or hydroquinone. The intriguing structures of the clavilactones and their significant biological properties have attracted the interest of chemists. Based on the efficient and stereoselective construction of α,β-epoxy-γ-butyrolactone skeleton, Prof. Li’s group established a general, concise, and efficient approach for synthesis of clavilactone family and its derivatives. For examples, the total synthesis of (+) clavilactone B was completed in 6 steps with 15.1 % yield, 7 steps with 14.9 % yield for (+) clavilactone A, and 7 steps with 15.5 % yield for (+) the proposed clavilactone D. Besides, Prof. Li found that neither the spectroscopic data of synthetic clavilactone D, namely the originally proposed structure of clavilactone D, nor its regioisomer matched exactly with those of the natural product. Therefore, they pointed out that the structure of clavilactone D was assigned wrong (Scheme 3, Angew. Chem. Int. Ed. 2014, 53, 4164-4167).
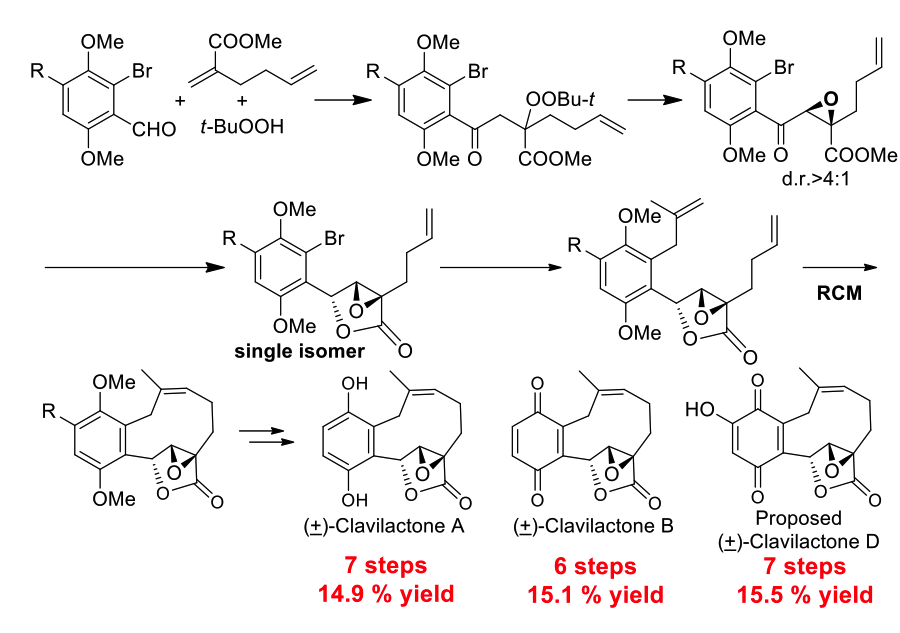
Scheme 3. Total synthesis of clavilactone family members
Recently, Prof. Li’s group reported the total synthesis and structure revision of (±)-clavilactone D. In the original isolation report (Phytochemistry 2000, 53, 1039-1041), it was suggested that m/z 302 was M+ from CIMS (CH4). This seems to be incorrect because chemical ionization with methane leads to MH+, not M+, so the hydroxyquinone should show an MH+ at 303. In the CIMS spectrum of clavilactone E an MH+ is observed as expected. MH+ for an amino quinone would be 302. Comprehensive analysis of the mass spectral and NMR spectroscopic data suggested that an amino group (-NH2) instead of a hydroxy group (-OH) was likely attached to the benzoquinone skeleton of clavilactone D. They report here the synthesis of aminobenzoquione and that both the mass spectral and NMR data are fully consistent with those of natural isolated clavilactone D (Scheme 4, J. Org. Chem. 2017, 82, 5487-5491).
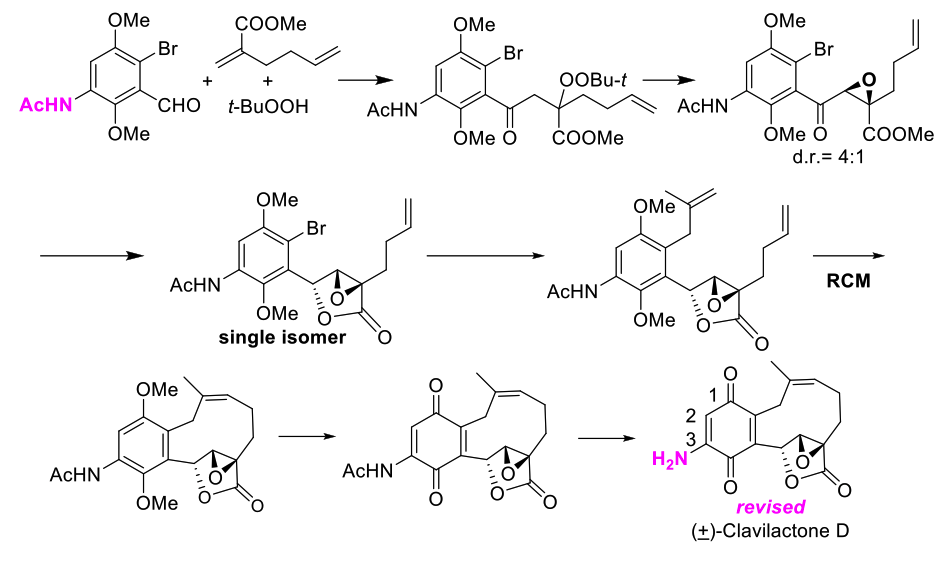
Scheme 4: Structure revision of (±)-clavilactone D